Product Description
Instant View® hCG Pregnancy Test Strips CLIA Waived
The Instant View hCG Pregnancy Test Strips CLIA Waived deliver rapid, accurate results in a convenient strip format. One Step hCG Urine Test is a rapid pregnancy test, which you can easily carry out yourself. It detects the presence of human chorionic gonadotropin (hCG), which appears in urine very early during pregnancy. Individual foil pouch.
- CLIA Waived
- FDA Cleared
- FDA 510K#: K984079
HOW DOES IT WORK?
Human chorionic gonadotropin (hCG) is a hormone, produced by the developing placenta shortly after the conception and secreted into the urine. The pregnancy test contains antibodies which specifically react with this hormone. When the strip is immersed into a urine specimen, capillary action carries the specimen to migrate along the membrane. When hCG in the sample reaches the Test Zone region of the membrane, it will form a colored line. Absence of this colored line suggests a negative result. To serve as a procedure control, a colored line will appear at the Control Zone region, if the test has been performed properly.
 Alfa Scientific Designs Fertility Products Flyer
Alfa Scientific Designs Fertility Products Flyer
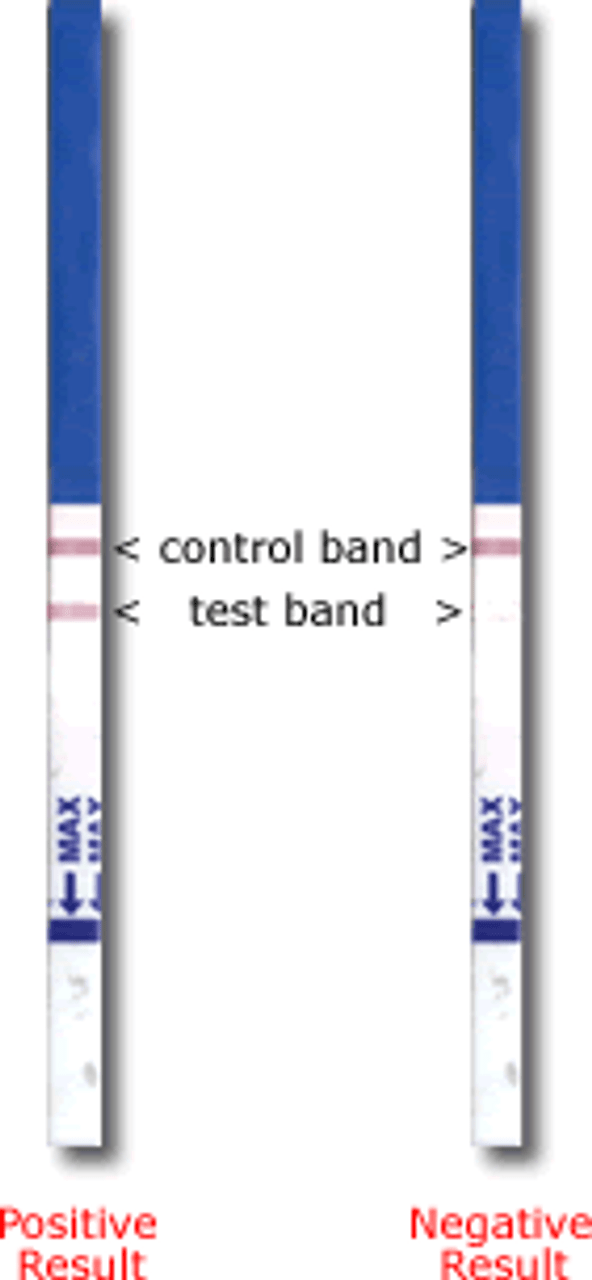
HOW TO READ RESULTS:
Negative (not pregnant) :
Only one color band appears in the Control Zone. No apparent band on the Test Zone. This indicates that no pregnancy has been detected.
Positive (pregnant) :
Distinct color bands appear in the Control and Test Zones. It indicates that you are pregnant. The color intensity of the test bands may vary since different stages of pregnancy have different concentrations of HCG hormone.
Invalid:
No visible band at all, or there is a visible band only in the test region and not in the control region. Repeat with a new test kit. If test still fails, please contact the distributor where you bought the product with the lot number.
Accurate results in just 6 to 8 days after conceiving - and days before your missed period
| Specifications | |
| Manufacturer # | 02-2487 |
| Brand | Instant-view® |
| Manufacturer | Alfa Scientific Designs Inc |
| Country of Origin | United States |
| Application | Reproductive Health Test Kit |
| CLIA Classification | CLIA Waived |
| CLIA Classified | CLIA Waived |
| Contents 1 | (50) Individually Pouched Test Devices with Dropper Pipette and Desiccant, Package Insert |
| Cutoff | 25 mIU / mL Cutoff |
| Number of Tests | 50 Tests |
| Reading Type | Visual Read |
| Sample Type | Urine Sample |
| Specialty | Immunoassay |
| Test Format | Dipstick Format |
| Test Kit Type | Rapid |
| Test Method | Lateral Flow Method |
| Test Name | hCG Pregnancy Test |
| Test Type | Fertility Test |
| Time to Results | 2 to 5 Minute Results |
| Kit Storage Conditions | Room temperature (15°C to 30°C/59°F to 86°F) |
| Sensitivity | 25 mIU/mL |
| Accuracy | >99% accurate and are capable of detecting human chorionic gonadotropin (hCG), at levels of just 25mIU/ml/hCG |
| Shelf Life | 24 months from date of manufacture |
Product Videos
Custom Field
Product Reviews
Orders received by 3:00 PM EST Monday through Friday ship the same day. Orders placed after 3:00 PM EST or on weekends and holidays will ship the next business day. Saturday delivery is available for select areas; orders must be placed by phone with Customer Service to arrange Saturday delivery.