Product Description
Healgen® Rapid Strep A Antigen Test
FDA 510(k) Cleared · CLIA Waived · 25 Tests/Box
- Qualitative detection of Group A Streptococcal antigen from throat swab.
- ~5 minute results, cassette format, no instrument required.
- Includes external positive & negative controls for QC.
Overview
The Healgen® Rapid Strep-A Antigen Test, FDA 510(k) (REF GCSTR-501Ca) is a lateral flow immunoassay for the qualitative detection of Group A Streptococcal antigen from throat swab specimens. It is FDA 510(k) cleared (K212623) and CLIA Waived for point-of-care use.
- Intended use: Aid in the diagnosis of Group A Streptococcal infection.
- Format: Cassette; specimen: human throat swab.
- No additional instrumentation required.
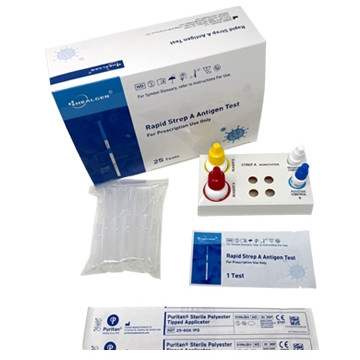
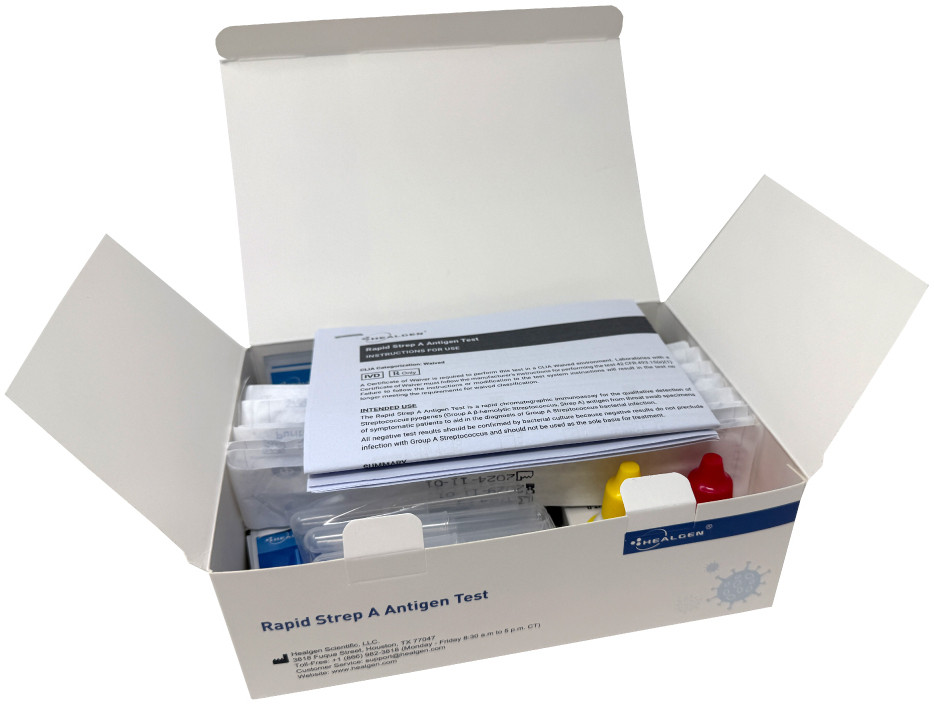
Kit Contents (25 Tests)
- 25 test cassettes
- 25 sterile throat swabs
- 25 extraction tubes + tube holders
- Extraction reagents:
- Reagent A (10 mL): 2.0 M Sodium Nitrite
- Reagent B (10 mL): 0.2 M Acetic Acid
- External controls: Positive (non-viable Strep A; 0.05% ProClin™ 300) and Negative (non-viable Strep C; 0.05% ProClin™ 300)
- Instructions for Use + Quick Reference Guide
Specifications
| Catalog / REF | GCSTR-501Ca |
| Time to Results | Approximately 5 minutes |
| Sensitivity / Specificity | 96.5% / 99.5% |
| Shelf Life | 24 months from date of manufacture |
| Storage | Room temperature |
| CLIA Status | Waived |
| Regulatory | FDA 510(k) cleared; 510(k) #: K212623 |
Clinical & Operational Notes
- Negative results should be confirmed by throat culture when clinical suspicion remains.
- Use only by healthcare professionals in settings operating under a CLIA Certificate of Waiver, Compliance, or Accreditation.
- Read the package insert (IFU) prior to use; follow all quality control procedures.
Product Videos
Custom Field
Product Reviews
Orders received by 3:00 PM EST Monday through Friday ship the same day. Orders placed after 3:00 PM EST or on weekends and holidays will ship the next business day. Saturday delivery is available for select areas; orders must be placed by phone with Customer Service to arrange Saturday delivery.